INSTITUTE OF PHILOSOPHY OF NATURE
Abstract
In the present concept, electrons in atomic system are considered identical with fixed mass and unit negative charge. But planets of solar system are not identical as they have different sizes and different mass. Again planets are considered neutral bodies whereas electrons carry electric charge. These features and many others made the atomic system different from solar system. But both atomic system and solar system are centrally organized systems having nucleus and extra-nuclear structure and they belong to the same universe. Therefore they are expected to have similar structural features and properties for uniformity of nature. This implies the electrons of atomic structure to have different mass like that of planets and planets to have different non-electric charge like the electric charge of electrons [1]. If mass only varies leaving charge then we would face problem to justify the deflection of electron in an electric field with varying mass of electrons. However this problem may not persist if the charge of electron varies in proportion to mass. We also find the galaxies have range of sizes; the stars have range of sizes; the planets have range of sizes; the satellites have range of sizes, the asteroids have range of sizes, the sand particles have range of sizes, the atomic nuclei have range of sizes and even the atom of one class has many isotopes. No two things are found exactly identical in this universe even the thumb impressions and DNA of human beings differ from one to other which helps to identify the specific person. This author feels perhaps by assuming identical nature of electrons (unit charge with fixed mass) modern science has deviated away from the reality of nature. A simple mental feeling has no place in science. Hence the author analyses the basic experiment that established the charge and mass of electrons. No experimental evidence can be challenged, however the interpretation of the experimental result can be argued upon. The charge of electron was determined by Millikan Oil Drop Experiment. Millikan found that the electric charge of the oil droplet was integer multiple of a common value. Millikan justified the above finding by assuming that the electrons are attached to the oil drop one by one, which produced the observed result and established the charge and mass of electron. This author argues that the surface of the oil drop in atomic scale has different nature of undulations (unevenness) which form different groups of cavities, each group having different space charge potential of the cavity. Thus electrons are attached to the oil drop in lot-by-lot depending on the levels of space charge potentials of surface cavities. Hence what we measure as the charge of single electron actually corresponds to the cumulative charge of an electron-lot. Within the electron-lot there is enough scope of variation of both mass and charge of each electron while maintaining the charge to mass ratio (e/m) of electron same for all electrons. The new fact of electron has scope of explaining different natural phenomena in a better manner as well as agrees with the uniformity of nature and further makes additional score on similarity between solar system and atomic system.
Key words: Atomic system, Solar system, Central force, Interaction ranges, e/m ratio, non-identical electron.
History of electric charge
Thomson was experimenting on electric currents inside vacuum glass tube and investigating on the long-standing puzzle known as “cathode ray”. He made a bold proposal that, cathode rays are streams of particles much smaller than atom they are in fact minuscule pieces of atoms. It was startling to imagine a particle residing inside atom, particularly, when the atom was considered indivisible and the most fundamental unit of matter. Thomson made a breakthrough in looking to the structure of a so-called structure-less atom. Cathode rays were material constituents of atoms, turned out to be correct. The rays are made up of electrons. Thomson showed that charge to mass ratio, e/m, of electron was independent of cathode material. He further showed that the negatively charged particles produced by radioactive materials, by heated materials and by illuminated materials were universal. His experiment measured the force on tiny charged droplets of oil suspended against gravity between two metal electrodes. Knowing the electric field, the charge on the droplet could be determined. Repeating the experiment for many droplets, Millikan showed that the results could be explained as integer multiples of a common value (1.592 × 10-19 coulomb). He then attributed the lowest charge of the oil drop as the charge of a single electron. This is somewhat lower than the modern value of 1.602 176 53(14) x 10-19 coulomb.
Electrons may not be identical
Millikan’s technique of measurement of electric charge of the oil droplet and finding the charge of the droplet as integer multiple of a common value is simply outstanding however this author argues that the lowest charge in the oil droplet is not due to the attachment of a single electron. The microscopic surface structure (surface atomic arrangement) of the oil the droplet reveals the possible sites of attachment of electrons. For visualizing the geometrical contour of the surface of the oil drop, the oil drop may have to be magnified by many orders. The smooth surface of the oil droplet in macro scale becomes highly uneven in microscopic atomic scale. The surface atoms of oil drop are arranged in different three-dimensional array (representing atoms of different kinds and in different position of lattice sites). In micro scale, the surface shows different pattern of symmetry forming different groups of cavities with different surface features of pits/cavities due to atomic arrangement. Fig. 1 shows the schematic view of the rough surface of the oil drop when magnified. The total surface of the oil droplet is formed by repetition of unit surface pattern. The unit surface contains one cavity of each potential level. The unit surface of the oil droplet is shown schematically in Fig. 2.
The uneven surface atomic arrangement of the oil drop forms different groups of charge potential cavities which promote selective electron attachment in lot-by-lot responding to different levels of charge potential. The charge potential cavities are the probable sites for electron attachment. All cavities do not have same potential level. The surface cavity potentials are classified into few groups depending on the cavity potential. The cavities of each group have one potential level and different groups of cavities have different potentials. Hence the electron attachment affinity can be grouped according to the charge potential levels of cavities. The higher level potential cavities have stronger affinity for electron attachment on the droplet. Depending on the surface molecular structure, different levels of potential wells (cavities) are formed. Each unit surface comprises one charge-potential-cavity from each potential level. Hence the surface of the oil drop has equal number of charge potential cavities in each potential level. The driving force for electron attachment is low for lower potential cavities. Thus electrons are attached first only at highest level of potential cavities. Only after filling up the cavities of highest potential level, the electron attachment takes place at next lower level of potential cavities and then electrons fill the next lower level of potential cavities. Let n be the number of sites (potential cavities) in each level. Hence electrons are attached on the oil droplet in steps of quantum jump as n, 2n, 3n or 4n. For this reason Millikan found the electric charge of the oil droplet as integer multiple of a common value which he erroneously attributed as the charge of a single electron.

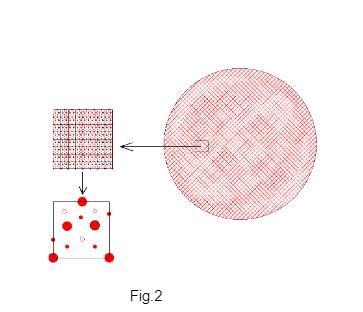
Unit surface contains representative surface cavities from each group of space potential cavities. The surface of oil droplet has n numbers of unit surfaces hence n number of charge potential cavities in each level.
It is possible that all free electrons in a given local background condition can have same e/m value. Thus the free electrons in a fixed background potential prevailing on the surface of the earth have the same e/m value. According to the new concept of gravity, particles with higher value of e/m move downward (into the ground) and those having lower value of (e/m) move upward. This phenomenon is easily understood from the new physical concept of charge and new basic interactions of mass and space [2]. Thus it is proper to assume that electrons have a range of sizes, charge and mass while their e/m values remain constant in a given background space potential, say that prevailing on the surface of the earth or at any fixed level of atmosphere. Each electron, large or small has the equal chance to respond to attachment in the potential cavity on the surface of the oil droplet.
Consider n number of cavities in each level of charge potential cavity. What Millikan assumed as one electron attachment in the oil droplet, in reality, corresponds to a lot of large number of electrons with different masses and charges having the same e/m values. The charge q of the oil drop with n number electrons with varying charge and mass is nearly same. Considering n number of electrons with different charges and masses filled up in each group of charge potential cavity. It is feasible to have different quantum charge-states of oil droplets in Millikan experiment even with the change of concept. Thus the charge states of oil droplets can have values as q, 2q, 3q … The group effect of real electrons on the surface of the droplet if assumed as the effect of a single electron then the hypothetical electron represent the cumulative charge and mass of real electrons.
To visualize the new concept, if one picks up say 1000 sand particles at random from a sand source and takes the weight he would find the weight of 1000 sand particles to be nearly same in every time he repeats his experiment. This experiment does not conclude that the sand particles are identical. Millikan oil drop experiment measures the charge of oil drop contributed by one lot of electrons but Millikan erroneously attributed the charge of oil drop as if contributed by a single electron. Thus the hypothesized electrons though considered identical but the real electrons have a range of size with varying mass and charge, however, the value of e/m remains constant for all electrons in the lot. A group of electrons having same e/m ratio exhibits identical deflection in a given electric field. This allows variation of both mass and charge of individual electron similar to variation in mass and charge of planets. A planet is one domain up in size of electrons and we have seen it carries celestial charge. This author has shown the existence of different non-electric charges in matters of other domains [1].
Mass of electron
The mass of electron is known to be 9.10933837 x 10-31kg. What is the significance of this accurate mass of electron when the present electron is the representative of large number of real electrons? We have simply assumed the charge of electron as one electronic charge and determined the mass to higher degree of accuracy from the experimental value of e/m. Greater is the accuracy of experimental finding of e/m greater is the accuracy of evaluated mass of electron. If now we make a different approach and assume the mass of electron is one basic mass unit then we get a scope to evaluate the charge of electron accurately from the experimental value of e/m and the assumed unit mass of electron. If the value of mass and charge change in proportion to one another then, the value of e/m remains invariant. We have no means to directly evaluate both e and m from the experimental value of e/m. We assumed electrons are identical and each carries unit charge. The mass of electron is evaluated based on above assumptions. Thus the mass of electron is not obtained from experiment. If one ignores the identical nature of electron and emphasize on e/m it opens up new avenue for variation of mass and charge of electron while keeping the experimental value of e/m of electron intact. By this new analysis, we are one step ahead in our march towards finding similarity between solar system and atomic system.
By assuming the mass of each electron same we fail to understand the difference between energy and energy-level of electron. The energy level decides the feasibility of a work function. If the work function is feasible only then energy comes into picture to know the extent of work done. The less known charge potential of electron (energy level of an electron) is decisive parameter whether a charge particle can overcome a charge field barrier but not the charge (energy). Without realizing this, we confuse how some electrons cross a field barrier with less energy than required to cross the barrier. To provide some reasoning for the above phenomenon scientists have accepted unscientific reasoning for tunneling of electron which states, ‘some electrons think the barrier is small and they have more energy to overcome the barrier which they do. How much we are justified in accepting this logic for tunneling of electrons in science?
Conclusion
The real electrons are of different sizes with different mass and charge. This new concept of electron is in the light of the uniformity of nature in different domains. One need not worry for how to deal with the physics and chemistry of matter when the well-established discrete quanta of charge and mass are changed! According to this author, if the defective concept of electron can explain some aspect of nature then the real electron can explain all aspects of nature. The new concept of electron helps to come out of the dogmatic concept of electron thereby helping scientists to explore many realities of nature.
Reference
- Eleceric and non-electric charges in matters of different domain (Page168), Dynamics of Universe interplay of matter, space and charge, vol.3. Published by Gyanajuga Publication, 2012.
- https://philosophyofnature.org.in/basic-constituents-of-universe-and-their-interactions.

thanks for info.